RESEARCH BRIEFING
Today is just like any other given Thursday: yesterday, 182,000 new coronavirus cases were reported in the United States.
That's not so important for Thanksgiving plans if we are being honest. Afterall, a typical infected person on Wednesday would likely not have a positive PCR for SARS-Cov-2 until Sunday or Monday, if not Tuesday, and people who do not yet test positive for the virus are not thought to be able spread it. The problem is not yesterday's infections, but those from last Saturday.
Many individuals who contracted the coronavirus around five days before today's festive meal are just now becoming contagious. Some of these people may have even been tested on Monday, Tuesday, or Wednesday, in an effort to protect any family they may be visiting for the holiday. But those tests would have been performed too soon, at times when the results would still be expected to be negative while the virus incubates inside the body. That means that many people who tried to do the right thing and get tested before traveling might still be delivering contagious virus to their loved ones today. If a test were taken today, many would be starting to shed enough virus to mount a positive test. Unfortunately, at-home tests of contagiousness have not yet become common and turnaround times for standard tests are slow.
Yes, many of these newly contagious cases will be asymptomatic. Some may be "pre-symptomatic," meaning they will develop symptoms of covid-19 sometime in the coming 3-7 days. But as we have come to know, these individuals are indeed contagious. They can and do spread the virus, though perhaps a bit less efficiently—the US Centers for Disease Control and Prevention now assumes that asymptomatic patients spread the virus around 75 percent as much as those with symptoms. Scientists also believe that those who are on the verge of having symptoms or who are just starting to show symptoms are at their most contagious.
The contagious period of SARS-CoV-2 is now thought to be under 10 days for most people. But that means around 1.5 million Americans are likely to be contagious at this very moment, of which, ballpark, over 300,000 will have become infected last weekend (and therefore become contagious in the 24 hours surrounding this post). That number rises if we extend the window to 36 hours before and after today's gatherings. Plus, there are those who have been contagious for longer, but do not realize it because of asymptomatic disease. Because of this, it is likely that a majority of contagious individuals do not realize that they are spreading virus.
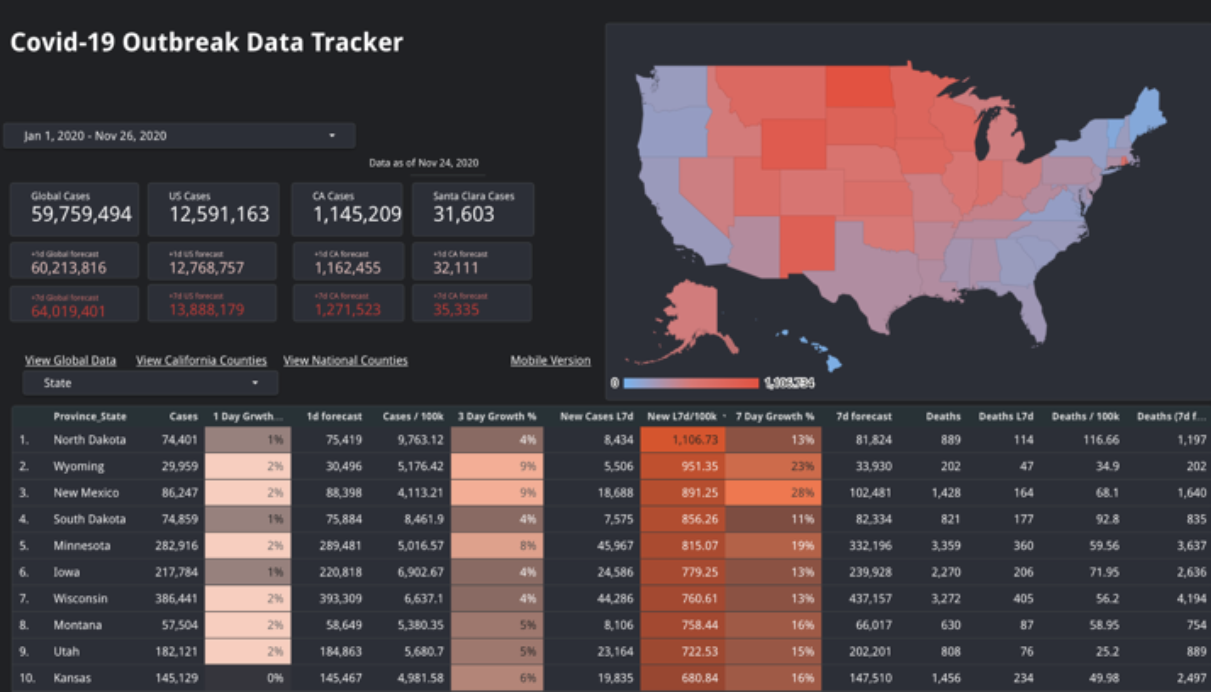
The typical ten-person Thanksgiving gathering poses a serious risk (for information on the risk in a ten-person event in your own state, click here). At the lower end of per capita cases (places like Oregon and Virginia), somewhere between one-in-10 and one-in-40 such events are expected to have at least one positive case present. At the high end, (North Dakota, Wyoming, New Mexico), the odds can even approach 50%, if we assume that many cases are being missed.
Nobody wants to be responsible for spreading covid-19. But today poses a risk that is statistically impossible to ignore. Plus, cases caught today won't become contagious until next week. And so the cycle continues.
As coronavirus vaccines are on the horizon and manufacturers have announced new results from phase III clinical trials via press release, it is important to distinguish the difference between vaccine efficacy and vaccine effectiveness. These terms are often incorrectly used interchangeably, as Health and Human Services Secretary Alex Azar said in a recent video that Pfizer's vaccine showed "95% effectiveness."
Clinical trial results and press releases point to vaccine efficacy — measurements made during a clinical trial. Trials are conducted under "idealized conditions" — all (or most) doses are administered on time, the vaccine is stored at proper temperatures, and subjects are selected based on specific and often narrow eligibility criteria. The resulting statistics from these trials are known as "vaccine efficacy." Efficacy is also known as a "direct" effect, which describes the individual measure of protection.
Vaccine effectiveness, on the other hand, describes how well the vaccine works in the real world. Based on previous vaccine trials, effectiveness may be somewhat lower than efficacy. Effectiveness is a broader term that defines how the vaccine performs in a population and includes "direct" and "indirect" effects. Indirect effects can include prevention of infection or reducing infectiousness within the population. As a result, vaccine effectiveness will vary based on "coverage" (how much of the population is vaccinated) and geographic location. In order to gather data on vaccine effectiveness, Phase IV trials (such as a cluster randomized trial of a typhoid vaccine) are conducted after vaccines have been licensed and are on the market, in order to ascertain population-level impact on the virus, bacteria, or other infectious disease.
(transcribed and adapted with permission by Benjy Renton)
Find it here and find out how your country, state, and county are doing.